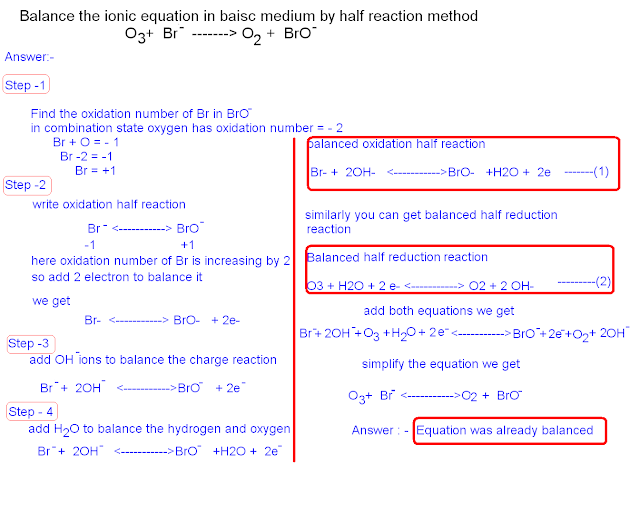
Answer:-
Step -1
Find the oxidation number of Br in BrO-
in combination state oxygen has oxidation number = - 2
Br + O = - 1
Br -2 = -1
Br = +1
Step -2
write oxidation half reaction
Br- <-----------> BrO-
-1 +1
here oxidation number of Br is increasing by 2
so add 2 electron to balance it
we get
Br- <-----------> BrO- + 2e-
Step -3
add OH - ions to balance the charge reaction
Br- + 2OH- <----------->BrO- + 2e
Step - 4
add H2O to balance the hydrogen and oxygen
balanced oxidation half reaction
Br- + 2OH- <----------->BrO- +H2O + 2e ---------------(1)
similarly you can get balanced half reduction reaction
Balanced reaction half reaction
O3 + H2O + 2 e- <-----------> O2 + 2 OH- ---------------(2)
add both equations we get
Br- + 2OH- + O3 + H2O + 2 e- <-----------> BrO- + 2e + O2 + 2 OH-
simplify the equation we get
O3+ Br- <----------->O2 + BrO-
Answer : - Equation was already balanced
 |
| Balance the ionic equation |






