A card is selected from a standard deck of 52 playing cards. A standard deck of cards has 12 face cards and four Aces (Aces are not face cards).
Find the probability of selecting ·
- a five given the card is a not a club.
- a heart given the card is red.
- a face card, given that the card is black.
Show step by step work. Give all solutions exactly in reduced fraction form
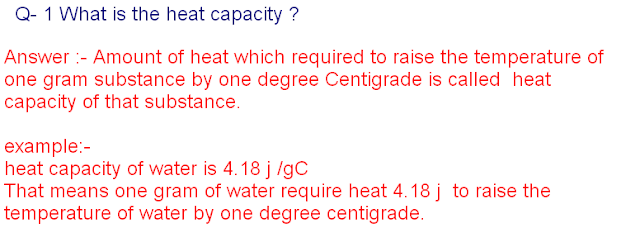
Answer : -
1) there are total 4 five cards but one of them is five of club
so number of 5 which is not club are 3
and there are total 13 clubs card
so non clubs card will 52- 13 will 39
formula of probability = favorable out comes / total out comes
so probability of a five given the card is a not a club. = 3/39
2) there are total 13 heart card
and total red cards are 26
so that probability of card is red
= 13/26
= 1/2
3) there are total 26 black card
there will 3 face card of spades (♠) and
3 face card of clubs (♣)
so total card will be 6 of spades and clubs
probability of a face card, given that the card is black
= favorable out comes / total out comes
=6/26
= 3/13