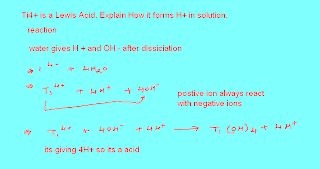
Friday, March 30, 2012
Thursday, March 29, 2012
Wednesday, March 28, 2012
Arrange the following elements in increasing reactivity.Cs, Ca, CI
Please send Email at nirapendra.singh@gmail.com to get the answer
an atop of hypothetical element of 31/15x consist of atom
The Atom is phosphorus represented by P
its atomic mass is 31 and atomic number is 15what will happen to the pressure of a gas in a container at constant temperature, if the volume of the container is changed to one-half its original size.
The pressure will increase to twice its original value.
Which of the following shows a correctly balanced reaction in which cerium is reduced?
Ce4+ (aq) + Fe2+ (aq) --> Ce3+ (aq) + Fe3+ (aq)
Subscribe to:
Comments (Atom)