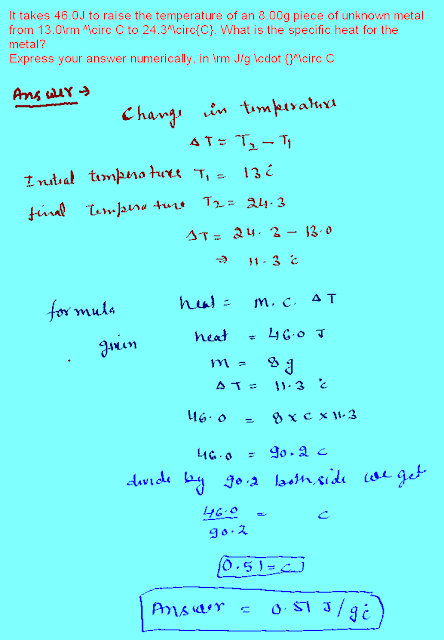
Tuesday, December 11, 2012
Tuesday, December 4, 2012
End point , Molarity ,pH value problems
If these solutions help you in your learning skill ,please join us and add us on Google plus.
Please click on problem to see answer
problem -1
The titration of 10.00 mL of a diprotic acid solution of unknown concentration requires 21.37 mL of a 0.1432 M NaOH solution. What is the concentration of the diprotic acid solution?
problem -2
The titration of 10.00 mL of a diprotic acid solution of unknown concentration requires 21.37 mL of a 0.1432 M NaOH solution. What is the concentration of the diprotic acid solution?
problem -3
A 0.2602 g sample of an unknown monoprotic acid requires 12.23 mL of 0.1298 M NaOH solution to reach the end point. what is the molecular mass of the acid?
problem -4
What
is the final pH after 1 drop (0.05 mL) of 6 M HCI is added to 1.0 L of
freshly prepared pure water that was originally at a pH of 7.0. Is there
a significant pH change?
problem - 5
What is the molarity of pure water at 20 C?
problem 6
Density table of water at one atm pressure
Thursday, November 29, 2012
How many of the following are pure compounds? sodium, sugar, oxygen, air, iron
How many of the following are pure compounds? sodium, sugar, oxygen, air, iron
so answer of this problem is sugar
Which of the following processes require(s) chemical methods?
a)Separating a homogeneous mixture into pure substances.
b)Separating a heterogeneous mixture into pure substances.
c)Distilling a saltwater mixture.
d)Breaking a compound into its constituent elements.
At least two of the above (a-d) require chemical methods
a)Separating a homogeneous mixture into pure substances. Its can involve both physical and chemical method for the separation
Which is an example of a homogeneous mixture?
a)vodka
b)oily water
c)soil (dust)
d)sodium chloride aluminum
in this problem Vodka is homogeneous mixture because in out of four option,only vodka has a uniform composition throughout
Answer :-
Sodium is a element
Sugar is a compound
Oxygen is also a element
Air is a mixture
Iron is element
so answer of this problem is sugar
Which of the following processes require(s) chemical methods?
a)Separating a homogeneous mixture into pure substances.
b)Separating a heterogeneous mixture into pure substances.
c)Distilling a saltwater mixture.
d)Breaking a compound into its constituent elements.
At least two of the above (a-d) require chemical methods
Answer :-
a)Separating a homogeneous mixture into pure substances. Its can involve both physical and chemical method for the separation
b)Separating a heterogeneous mixture into pure substances. IT involve physical method only
c)Distilling a saltwater mixture it is a physical method
d)Breaking a compound into its constituent elements. this is a chemical reaction
so Answer is a and d
Which is an example of a homogeneous mixture?
a)vodka
b)oily water
c)soil (dust)
d)sodium chloride aluminum
in this problem Vodka is homogeneous mixture because in out of four option,only vodka has a uniform composition throughout
Assume the equilibrium constant for the reaction of a particular alcohol with a carboxylic acid is 7.87. What is the calculated yield of the ester?
Assume the equilibrium constant for the reaction of a particular alcohol with a carboxylic acid is 7.87. What is the calculated yield of the ester?
Assume the equilibrium constant for the reaction of a particular alcohol with a carboxylic acid is 7.87. What is the calculated yield of the ester?
If you want answer of this problem please mail me on nirapendra.singh@gmail.com
Assume the equilibrium constant for the reaction of a particular alcohol with a carboxylic acid is 7.87. What is the calculated yield of the ester?
If you want answer of this problem please mail me on nirapendra.singh@gmail.com
A calorimeter contains 35.0mL of water at 15.0 degrees celcius . When 1.20g of X (a substance with a molar mass of 51.0g/mol ) is added, it dissolves via the reaction X(s)+H2O(l)--->X(aq) and the temperature of the solution increases to 29.0 degrees celcius . Calculate the enthalpy change,(deltaH), for this reaction per mole of X. Assume that the specific heat and density of the resulting solution are equal to those of water [4.18 (J/g(degree celcius)) and 1.00g/mL ] and that no heat is lost to the calorimeter itself, nor to the surroundings.
Prob -1
A calorimeter contains 35.0mL of water at 15.0 degrees celcius . When 1.20g of X (a substance with a molar mass of 51.0g/mol ) is added, it dissolves via the reaction
X(s)+H2O(l)--->X(aq)
and the temperature of the solution increases to 29.0 degrees celcius .
Calculate the enthalpy change,(deltaH), for this reaction per mole of X.
Assume that the specific heat and density of the resulting solution are equal to those of water [4.18 (J/g(degree celcius)) and 1.00g/mL ] and that no heat is lost to the calorimeter itself, nor to the surroundings.
In this problem heat is consumed in increasing the temperature of water
heat Q = M*C* delta T
M = mass of water = 35.0 ml * 1 gram / 1 ml = 35 gram (density of water is 1gram per cm ^ 3 or 1.00g/mL )
delta T = final temperature - initial temperature
= 29 - 15 = 14 `C
specific heat capacity C = 4.18 (J/g(degree celcius)
put these value in equation we get heat Q = M*C* delta T
Heat Q = 35 * 4.18*14
= 2048.2j
When 1.20g of X (a substance with a molar mass of 51.0g/mol ) is added
number of moles of substance X = Mass / molar mass
= 1.20/51.0
= 0.0235 mol
Molar heat capacity = heat required / number of moles
= 2048.2 j / 0.035 mol
= 87048.5 jules (1000 j = 1 kj )
convert in kilojoules
= 87048.5/ 1000 kj
=87.05 kj
A calorimeter contains 35.0mL of water at 15.0 degrees celcius . When 1.20g of X (a substance with a molar mass of 51.0g/mol ) is added, it dissolves via the reaction
X(s)+H2O(l)--->X(aq)
and the temperature of the solution increases to 29.0 degrees celcius .
Calculate the enthalpy change,(deltaH), for this reaction per mole of X.
Assume that the specific heat and density of the resulting solution are equal to those of water [4.18 (J/g(degree celcius)) and 1.00g/mL ] and that no heat is lost to the calorimeter itself, nor to the surroundings.
In this problem heat is consumed in increasing the temperature of water
heat Q = M*C* delta T
M = mass of water = 35.0 ml * 1 gram / 1 ml = 35 gram (density of water is 1gram per cm ^ 3 or 1.00g/mL )
delta T = final temperature - initial temperature
= 29 - 15 = 14 `C
specific heat capacity C = 4.18 (J/g(degree celcius)
put these value in equation we get heat Q = M*C* delta T
Heat Q = 35 * 4.18*14
= 2048.2j
When 1.20g of X (a substance with a molar mass of 51.0g/mol ) is added
number of moles of substance X = Mass / molar mass
= 1.20/51.0
= 0.0235 mol
Molar heat capacity = heat required / number of moles
= 2048.2 j / 0.035 mol
= 87048.5 jules (1000 j = 1 kj )
convert in kilojoules
= 87048.5/ 1000 kj
=87.05 kj
Problem - 2 This one problem has me confused
I need 60.0 in scientific notation
is it 6.0x10^1 ??
I need 60.0 in scientific notation
is it 6.0x10^1 ??
your answer is correct
in scientific notation we always place decimal point after one digit from the left
example
60000000 = 6.0 x10^7
0.00006 = 6.0 x10^ -5
and 60.0 = 6.0x10^1 = 6.0x10
example
60000000 = 6.0 x10^7
0.00006 = 6.0 x10^ -5
and 60.0 = 6.0x10^1 = 6.0x10
Monday, November 19, 2012
Monday, November 5, 2012
Friday, November 2, 2012
Tuesday, October 23, 2012
Thursday, October 18, 2012
Monday, August 6, 2012
Saturday, June 30, 2012
Friday, June 8, 2012
If the speed of light is 3.0 ×10^8 m s-1, calculate the distance covered by light in 2.00 ns
Q-22 If the speed of light is 3.0 ×10^8 m s-1, calculate the distance covered by light in 2.00 ns.
Answer
According to the question:
Answer
According to the question:
1ns = 10^-9 s (conversion )
Time taken to cover the distance = 2.00 ns
= 2.00 × 10^–9 sSpeed of light = 3.0 × 10^8 ms–1
Distance= Speed of light × Time taken (formula )
= (3.0 × 10^8 ms –1) (2.00 × 10^–9 s)
= 6.00 × 10^–1 m
= 0.600 m
Thursday, June 7, 2012
The following data are obtained when dinitrogen and dioxygen react together to form different compounds: Mass of dinitrogen Mass of dioxygen (i) 14 g 16 g (ii) 14 g 32 g (iii) 28 g 32 g (iv) 28 g 80 g
Q- 21 The following data are obtained when dinitrogen and dioxygen react together to form
different compounds:
Mass of dinitrogen Mass of dioxygen
(i) 14 g 16 g
(ii) 14 g 32 g
(iii) 28 g 32 g
(iv) 28 g 80 g
Answer :-
(a) Which law of chemical combination is obeyed by the above experimental data?Give
its statement.
(b) Fill in the blanks in the following conversions:
(i) 1 km = ...................... mm = ...................... pm
(ii) 1 mg = ...................... kg = ...................... ng
(iii) 1 mL = ...................... L = ...................... dm3

different compounds:
Mass of dinitrogen Mass of dioxygen
(i) 14 g 16 g
(ii) 14 g 32 g
(iii) 28 g 32 g
(iv) 28 g 80 g
Answer :-
(a) Which law of chemical combination is obeyed by the above experimental data?Give
its statement.
(b) Fill in the blanks in the following conversions:
(i) 1 km = ...................... mm = ...................... pm
(ii) 1 mg = ...................... kg = ...................... ng
(iii) 1 mL = ...................... L = ...................... dm3
Answer :-
A) Fixing the mass of dinitrogen to 28g , mass of dioxygen combined with 32,64,32, and 80 g in the given four oxides . This is the ratio of 1:2:1:5 which is a simple whole number ratio . Hence the given data follow the law of multiple proportions
Law of Multiple Proportions This law was proposed by Dalton in 1803.According to this law, if two elements cancombine to form more than one compound, themasses of one element that combine with afixed mass of the other element, are in theratio of small whole numbers.For example, hydrogen combines with oxygen to form two compounds, namely, water and hydrogen peroxide.
Hydrogen + Oxygen → Water
2g 16g 18g
Hydrogen + Oxygen → Hydrogen Peroxide
2g 32g 34g
Here, the masses of oxygen (i.e. 16 g and 32 g)which combine with a fixed mass of hydrogen (2g) bear a simple ratio, i.e. 16:32 or 1: 2. .
Hydrogen + Oxygen → Water
2g 16g 18g
Hydrogen + Oxygen → Hydrogen Peroxide
2g 32g 34g
Here, the masses of oxygen (i.e. 16 g and 32 g)which combine with a fixed mass of hydrogen (2g) bear a simple ratio, i.e. 16:32 or 1: 2. .
B)
Subscribe to:
Comments (Atom)